Asked by Briana Bradley on May 10, 2024

Verified
Classify the compound below as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network. 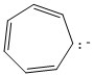
AntiAromatic
Refers to a cyclic, conjugated compound with an odd number of pairs of pi electrons that does not follow Huckel's rule, often making it less stable.
π Network
A system of connected π bonds, allowing delocalized electron movement across molecule segments.
- Analyze the structure of compounds to categorize them as aromatic, antiaromatic, or nonaromatic.
- Attain an understanding of the concept of planarity and its effect on the aromatic quality of a compound.

Verified Answer
ZK

Learning Objectives
- Analyze the structure of compounds to categorize them as aromatic, antiaromatic, or nonaromatic.
- Attain an understanding of the concept of planarity and its effect on the aromatic quality of a compound.
Related questions
Which of the Following Structures, If Flat, Would Be Classified ...
Classify Cyclopentadienyl Cation as Aromatic, Antiaromatic, or Nonaromatic ...
Classify the Compound Below as Aromatic, Antiaromatic, or Nonaromatic ...
Classify the Compound Below as Aromatic, Antiaromatic, or Nonaromatic ...
Classify Naphthalene as Aromatic, Antiaromatic, or Nonaromatic ...