Asked by Sofia Uzquiano on Jun 03, 2024

Verified
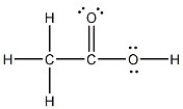
Draw the Lewis structure of acetic acid, CH3CO2H, including all non-bonding lone pairs.
Lewis Structure
A diagram that represents the valence electrons of atoms within a molecule, illustrating the bonding between atoms and the existence of lone pairs of electrons.
Acetic Acid
A weak, organic acid that gives vinegar its characteristic smell and sour taste; used as a chemical reagent.
Lone Pairs
Electron pairs in the outer shell of an atom that are not involved in chemical bonding.
- Diagram Lewis structures to showcase both bonding interactions and non-bonding lone pairs.

Verified Answer
ZK

Learning Objectives
- Diagram Lewis structures to showcase both bonding interactions and non-bonding lone pairs.