Asked by Keayanne Harmon on Sep 24, 2024

Verified
Which of the following are intermediates in the acid hydrolysis of an amide? 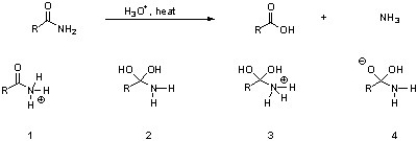
A) 1
B) 2
C) 2 & 3
D) 4
E) 1, 2, & 3
Acid Hydrolysis
A process where water and an acid are used to break down compounds, often polymers or esters, into smaller units.
Amide
An organic compound characterized by a carbonyl group (C=O) attached to a nitrogen atom.
- Identify intermediates in the hydrolysis of amides.

Verified Answer
JB
Jessica Bernik4 days ago
Final Answer :
C
Explanation :
The acid hydrolysis of an amide involves the reaction with an acid and water to form a carboxylic acid and an amine. The intermediates in this reaction are the protonated amide (2) and the tetrahedral intermediate (3), which is formed when water attacks the carbonyl carbon of the protonated amide. Therefore, the correct choice is C (2 & 3).

Learning Objectives
- Identify intermediates in the hydrolysis of amides.