Asked by Chris Adams on Sep 25, 2024

Verified
Consider the substitution reaction shown below. By what mechanism will the reaction proceed? 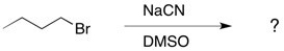
A) SN1
B) SN2
C) free radical reaction
D) There is not enough information to tell.
Substitution Reaction
A chemical reaction where one functional group in a chemical compound is replaced by another.
Mechanism
A mechanism is a description of the step-by-step process by which reactants are transformed into products in a chemical reaction, including the intermediate stages and transition states.
- Develop reactions employing distinct methodologies (e.g., SS1U1B1NS1U1B02) and forecast the architecture of significant organic products.

Verified Answer
BS
Briana Smith2 days ago
Final Answer :
B
Explanation :
The reaction involves a nucleophilic substitution at a sp3 hybridized carbon. Specifically, an alkyl halide (R-X) is reacting with a nucleophile (OH-) to form an alcohol (R-OH). This type of reaction most commonly proceeds through an SN1 or SN2 mechanism. Based on the nature of the substrate and the nucleophile used in this reaction, it is most likely to proceed through an SN2 mechanism (SN2).

Learning Objectives
- Develop reactions employing distinct methodologies (e.g., SS1U1B1NS1U1B02) and forecast the architecture of significant organic products.