Asked by Stella Grace on Sep 26, 2024

Verified
Which atomic orbital combination would result in a molecular sigma bond?
A) 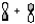
B) 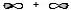
C) 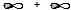
D) 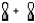
Sigma Bond
A single covalent bond formed from the direct overlap of atomic orbitals, sharing a pair of electrons between atoms.
Atomic Orbital
A mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom, defining their probability distribution around a nucleus.
- Comprehend the development and features of sigma and pi bonds.

Verified Answer
JB
Jessica Bernik1 day ago
Final Answer :
B
Explanation :
Molecular sigma bond is formed by the overlap of two atomic orbitals along the internuclear axis. In option B, the two atomic orbitals are overlapping along the internuclear axis resulting in a molecular sigma bond.

Learning Objectives
- Comprehend the development and features of sigma and pi bonds.
Related questions
Which of the Following Statements About π Molecular Orbitals Is/are ...
Which of the Following Statements Best Describes the Relative Bond ...
Acrylonitrile (CH 2 =CHCN) Contains ________ σ Bonds and ________ π Bonds ...
If a Compound, C 5 H 7 NO, Contains 1 Ring, How Many Pi ...
How Many π Bonds Are Present in the Molecule Shown ...