Asked by Braxton Graves on Apr 26, 2024

Verified
Assign the correct formal charge to each nitrogen atom in the following Lewis structure. (All non-bonding electrons are included.) 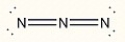
Formal Charge
A concept in chemistry that represents the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between atoms, irrespective of electronegativity.
Nitrogen Atoms
Atoms that have five electrons in their outer shell and are essential components of amino acids, proteins, and nucleic acids.
- Acquire the ability to calculate and understand formal charges in molecular structures.

Verified Answer
KF

Learning Objectives
- Acquire the ability to calculate and understand formal charges in molecular structures.