Asked by Lyntrell Butler on Jun 03, 2024

Verified
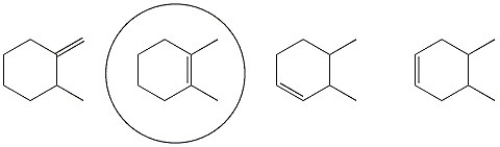
Circle the alkene below which has the smallest heat of hydrogenation. 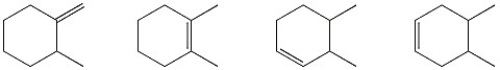
Heat Of Hydrogenation
The heat of hydrogenation is the change in enthalpy that occurs when an unsaturated compound reacts with hydrogen to become saturated, used as a measure of the stability of the compound’s double bonds.
Alkene
A hydrocarbon compound characterized by the presence of at least one carbon-carbon double bond, playing a vital role in organic chemistry.
- Explain the structural attributes that affect the stability of alkenes, focusing on cis-trans isomerism.

Verified Answer
NS

Learning Objectives
- Explain the structural attributes that affect the stability of alkenes, focusing on cis-trans isomerism.
Related questions
Which of the Following Is the Best Reaction Sequence to ...
What Synthetic Goal Is Achieved by Subjecting an Alkene to ...
What Is the Correct Molecular Formula for Methylpropane
Draw for Yourself the Lewis Structure of Ethane, C 2 H 4 , Also ...
How Many Carbon Atoms Should Be in the Molecule 2-Pentyne