Asked by Adrian Perry on Jun 03, 2024

Verified
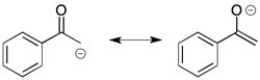
Draw the important resonance forms for the enolate ion/s that are formed when acetophenone reacts with base.
Resonance Forms
Different Lewis structures for a single molecule that cannot be represented by only one structure but by the hybrid of these structures, showing delocalized electrons.
Enolate Ion
A negatively charged ion formed by the deprotonation of an alpha carbon to a carbonyl group in an aldehyde or ketone.
Acetophenone
Acetophenone is an aromatic ketone, known as the simplest aromatic ketone, used as a precursor in the synthesis of fragrances and resins.
- Pinpoint and clarify the properties and relevance of tautomers, notably ketone-enol tautomerism.
- Gain insight into the enolization mechanism and the specific conditions promoting it.

Verified Answer

Learning Objectives
- Pinpoint and clarify the properties and relevance of tautomers, notably ketone-enol tautomerism.
- Gain insight into the enolization mechanism and the specific conditions promoting it.
Related questions
The Reaction of LDA with Acetophenone Produces ________ ...
When a Ketone and Its Enol Are in Equilibrium, Under ...
The Relationship Between Ketones and Their Corresponding Enols Is One ...
Prove the Following Claim ...
Which of the Following Correctly Lists the Conformations of Cis-1,4-Di-T-Butylcylcohexane ...