Asked by Misbah Siddiqui on Sep 28, 2024
Structures ________, shown below, are resonance structures, and structure ________ is the major contributor to the overall resonance hybrid. 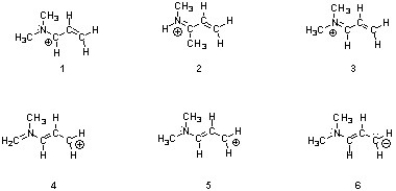
A) 2 & 4; 2
B) 1, 3 & 5; 3
C) 4 & 6; 6
D) 1, 3 & 5; 1
E) 1, 3, 4 & 5; 3
Resonance Structures
Representations of a molecule showing different electron distributions, but the actual molecule is a hybrid of these.
Major Contributor
The most significant structure or mechanism among several possibilities in a chemical reaction or process.
Resonance Hybrid
A representation of a molecule where the electron distribution is a hybrid of two or more structures, illustrating the delocalization of electrons.
- Apprehend and delineate the significance of resonance in the stabilization of molecular entities.
- Gain insight into the notion of formal charge and its computation related to the architecture of molecules.

Learning Objectives
- Apprehend and delineate the significance of resonance in the stabilization of molecular entities.
- Gain insight into the notion of formal charge and its computation related to the architecture of molecules.
Related questions
When a Molecule Can Best Be Represented as a Series ...
Which of the Following Structures (A-D) Is Another Resonance Structure ...
Nitroamines Are Common Functional Groups Found in Energetic Materials, Such ...
When a Negatively Charged Species Is Most Appropriately Depicted as ...
Provide the Major Resonance Structures of the Ion Which Results ...