Asked by Dejia Crocket on Sep 25, 2024
When (cis) -1-bromo-2 methylcyclohexane is treated with methanol and heat, four different products are formed - two by substitution and two by elimination. 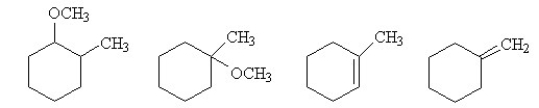 Which of the following conditions would change the outcome of this reaction by promoting the production of 3-methylhexene as the major product?
Which of the following conditions would change the outcome of this reaction by promoting the production of 3-methylhexene as the major product? 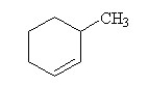
A) KOH, ethanol and heat
B) water/acetone and heat
C) tert-butoxide/tert-butyl alcohol
D) tert-butyl alcohol and heat
E) methoxide/methanol
Methanol
The simplest alcohol, with the chemical formula CH3OH, used as a solvent, antifreeze, fuel, and in the production of formaldehyde.
Ethanol
A two-carbon alcohol (C2H5OH) commonly used as a solvent, fuel, and in alcoholic beverages.
Methylhexene
An alkene with a methyl group and a hexene (six carbon linear chain with a double bond) component in its structure.
- Describe how reaction conditions influence the selection between substitution and elimination outcomes.

Learning Objectives
- Describe how reaction conditions influence the selection between substitution and elimination outcomes.
Related questions
Heating A(n) ________ Results in a Cope Elimination ...
The Hofmann Elimination Proceeds Via A(n) ________ Pathway ...
The Diels-Alder Reaction Is a Concerted Reaction; This Means ________ ...
Provide the Structure of the Major Organic Product in the ...
In Which of the Following Mechanisms (S N 1, S N 2, E1, E2) ...