NS
Natalie Smalls
Answers (14)
NS
Answered
What term describes the structural relationship between (2R,3R,4S) -2,3,4-trichloroheptane and (2S,3S,4R) -2,3,4-trichloroheptane?
A) not isomers
B) constitutional isomers
C) enantiomers
D) diastereomers
E) conformers
A) not isomers
B) constitutional isomers
C) enantiomers
D) diastereomers
E) conformers
On Aug 05, 2024
C
NS
Answered
When methanol (CH3OH) acts as a base, its conjugate acid is ________.
A) . -CH2OH
B) CH3O-
C) CH4OH
D) CH3OH2+
E) CH4O+
A) . -CH2OH
B) CH3O-
C) CH4OH
D) CH3OH2+
E) CH4O+
On Jul 11, 2024
D
NS
Answered
Bromine in the gaseous state is Br2 and has a simple Lewis structure. What type of bonding will it have?
A) A double, ionic bond
B) A single, ionic bond
C) A double, covalent bond
D) A single, covalent bond
A) A double, ionic bond
B) A single, ionic bond
C) A double, covalent bond
D) A single, covalent bond
On Jul 09, 2024
D
NS
Answered
Rank the following bonds in order of increasing stretching frequency (cm-1) in IR spectroscopy: 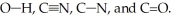
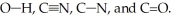
On Jun 09, 2024
C-N < C=O < C  N < O-H
N < O-H
 N < O-H
N < O-HNS
Answered
What is the hybridization of the positively charged carbon in (CH3)3C+?
On Jun 04, 2024
sp2
NS
Answered
The positively polarized carbon atom of a carbonyl group acts as ________.
A) an electrophile and a Lewis base
B) a nucleophile and a Lewis base
C) an electrophile and a Lewis acid
D) a nucleophile and a Lewis acid
E) both a Lewis acid and a Lewis base
A) an electrophile and a Lewis base
B) a nucleophile and a Lewis base
C) an electrophile and a Lewis acid
D) a nucleophile and a Lewis acid
E) both a Lewis acid and a Lewis base
On Jun 02, 2024
C
NS
Answered
What is a saturated solution?
A) one that contains the maximum amount of solute that can be dissolved
B) one that contains the maximum amount of solvent that can be dissolved
C) one that contains the maximum amount of solution that can be dissolved
D) one that contains the maximum amount of a salt that can be dissolved
A) one that contains the maximum amount of solute that can be dissolved
B) one that contains the maximum amount of solvent that can be dissolved
C) one that contains the maximum amount of solution that can be dissolved
D) one that contains the maximum amount of a salt that can be dissolved
On May 31, 2024
A
NS
Answered
In the lowest energy conformation of the compound below, how many alkyl substituents are equatorial? 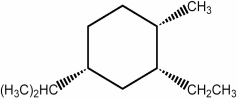
A) 0
B) 1
C) 2
D) 3
E) 6

A) 0
B) 1
C) 2
D) 3
E) 6
On May 28, 2024
C
NS
Answered
The hydrogen atom abstraction step in the free radical chlorine of methane is exothermic. Use the Hammond Postulate to speculate on the extent of bond formation and bond cleavage in the transition state.
On May 02, 2024
The exothermic nature of this step means that the energy of the transition state is closer to that of the step's reactants than to its products. Using the Hammond Postulate this means the transition state more closely resembles a chlorine radical than a carbon radical. The carbon-hydrogen bond has broken very little and the hydrogen-chlorine bond has formed very little in the transition state.
NS
Answered
Free radical bromination of pentane results in poor yields of 1-bromopentane, while cyclopentane can be readily brominated under similar conditions to yield bromocyclopentane. Offer an explanation.
On May 02, 2024
In the bromination of pentane, the lowest energy reaction pathways go through secondary free radical intermediates to produce secondary alkyl bromides (2-bromopentane and 3-bromopentane). Thus 1-bromopentane is a very minor product. All of the hydrogen atom abstractions of cyclopentane lead to the same secondary radical which eventually leads to bromocyclopentane.