Asked by Ahmed Ismail on Jun 09, 2024

Verified
Pyrene has been tentatively identified in the interstellar medium. Use Huckel Rule to determine is pyrene is aromatic or antiaromatic (assuming planarity of the π system). 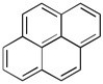
Pyrene
A polycyclic aromatic hydrocarbon (PAH) consisting of four fused benzene rings, resulting in a flat aromatic system.
Huckel Rule
A rule that helps predict the aromaticity of planar rings based on the count of pi electrons, stating that aromatic compounds must have 4n+2 pi electrons.
Aromatic
Refers to molecules that contain one or more planar rings of atoms with delocalized pi electron systems, typically associated with increased stability.
- Apply Huckel's rule to determine the aromaticity of polycyclic compounds.

Verified Answer
ZK
Zybrea KnightJun 12, 2024
Final Answer :
If you consider only the π electrons in the outermost ring, the number of π electrons is 14 which satisfied Huckels Rule and the compound is aromatic. 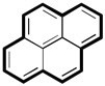


Learning Objectives
- Apply Huckel's rule to determine the aromaticity of polycyclic compounds.