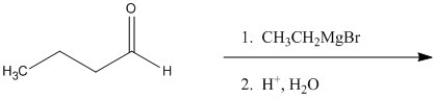NS
Nadia Sanchez
Answers (10)
NS
Answered
An ionic compound made from bromine and magnesium has what correct formula?
On Jun 21, 2024
MgBr2
NS
Answered
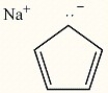
Circle the alkene below which has the smallest heat of hydrogenation. 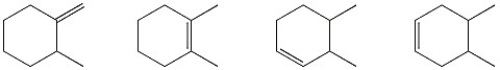

On Jun 05, 2024
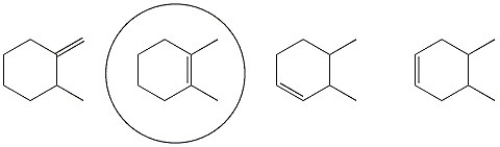
NS
Answered
What reagent is needed to convert CH3(CH2)14CH2OH to CH3(CH2)14CHO?
On May 28, 2024
PCC (pyridinium chlorochromate)
NS
Answered
Of the functional groups on various organic molecules that have been studied in this chapter, which can function as a base?
A) Ethers
B) Esters
C) Amines
D) Carboxylic acids
A) Ethers
B) Esters
C) Amines
D) Carboxylic acids
On May 16, 2024
C
NS
Answered
What synthetic goal is achieved by subjecting an alkene to an oxymercuration-demercuration sequence?
A) Markovnikov addition of H2O wherein skeletal rearrangement is promoted
B) Markovnikov addition of H2O wherein skeletal rearrangement is prevented
C) anti-Markovnikov addition of H2O wherein skeletal rearrangement is promoted
D) anti-Markovnikov addition of H2O wherein skeletal rearrangement is prevented
E) syn-hydroxylation
A) Markovnikov addition of H2O wherein skeletal rearrangement is promoted
B) Markovnikov addition of H2O wherein skeletal rearrangement is prevented
C) anti-Markovnikov addition of H2O wherein skeletal rearrangement is promoted
D) anti-Markovnikov addition of H2O wherein skeletal rearrangement is prevented
E) syn-hydroxylation
On May 02, 2024
B
NS
Answered
Why can methyl acrylate (H2C 
CHCO2CH3) be polymerized through anionic polymerization?

CHCO2CH3) be polymerized through anionic polymerization?
On May 01, 2024
The intermediate carbanion and the transition state leading to it are stabilized by the electron-withdrawing capacity of the carbonyl group.
NS
Answered
What happens to a gas phase ion in a magnetic field?
On Apr 29, 2024
It can be deflected.
NS
Answered
The pKa1 for oxalic acid (pKa = 1.27) is much lower than the pKa1 of glutaric acid (pKa = 4.35). Briefly explain why.
On Apr 28, 2024
The second carboxylic acid in oxalic acid is an electron withdrawing group and much closer than the carboxylic acid in glutaric acid. The electron withdrawing group causes the carboxylic acid to be more acidic.