ZK
Zybrea Knight
Answers (12)
ZK
Answered
What technique is often coupled with a radioimmunoassay to determine that a drug is present in a person?
A) Thin layer chromatography / mass spectrometry
B) Gas chromatography
C) Gas chromatography / mass spectrometry
D) Mass spectrometry
A) Thin layer chromatography / mass spectrometry
B) Gas chromatography
C) Gas chromatography / mass spectrometry
D) Mass spectrometry
On Jul 28, 2024
C
ZK
Answered
A polypeptide was treated with a certain enzyme to yield the following amino acid sequences. What enzyme was used to induce the observed fragmentations?
Val-Pro-Phe Leu-Ser-Lys-Glu-Trp Arg-Ile-Ser-Ser-Leu-Tyr
Val-Pro-Phe Leu-Ser-Lys-Glu-Trp Arg-Ile-Ser-Ser-Leu-Tyr
On Jul 18, 2024
Chymotrypsin
ZK
Answered
In general, when an aqueous solution is heated, what happens to the solubility of ionic compounds within it?
A) Solubility decreases.
B) Solubility increases.
C) Kinetic energy is added by the solute.
D) Kinetic energy is removed by the solute.
A) Solubility decreases.
B) Solubility increases.
C) Kinetic energy is added by the solute.
D) Kinetic energy is removed by the solute.
On Jun 21, 2024
B
ZK
Answered
PETN is the acronym for what explosive?
A) Pentaethylene glycol
B) Phospho-nitroglycerin
C) Phosphor-ethyl tetranitrate
D) Pentaerythritol tetranitrate
A) Pentaethylene glycol
B) Phospho-nitroglycerin
C) Phosphor-ethyl tetranitrate
D) Pentaerythritol tetranitrate
On Jun 17, 2024
D
ZK
Answered
Give the IUPAC name for the cycloalkane shown below. 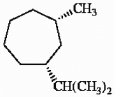

On Jun 11, 2024
cis-1-isopropyl-3-methylcycloheptane
ZK
Answered
Which of the following terms best describes the exterior surface of a soap micelle in water?
A) hydrophobic
B) hydrophilic
C) hard
D) saponified
E) hydrogenated
A) hydrophobic
B) hydrophilic
C) hard
D) saponified
E) hydrogenated
On Jun 09, 2024
B
ZK
Answered
When the volume of a gas decreases, what happens to its temperature if all other factors remain unchanged?
A) It remains constant.
B) It increases.
C) It decreases.
D) A pressure value is also needed to answer this.
A) It remains constant.
B) It increases.
C) It decreases.
D) A pressure value is also needed to answer this.
On May 16, 2024
C
ZK
Answered
What value in an equilibrium reaction does K have if the other values for the reaction HC2H3O2 ⇌ H+ + C2H3O2- are [A-] = 0.10 M and [HC2H3O2] = 0.25 M?
A) 0.01
B) 0.04
C) 0.03
D) 0.25
A) 0.01
B) 0.04
C) 0.03
D) 0.25
On May 15, 2024
B
ZK
Answered
What kind of oxygen balance does RDX have?
A) Positive
B) Negative
C) Neutral
D) Not enough information to tell
A) Positive
B) Negative
C) Neutral
D) Not enough information to tell
On May 12, 2024
B
ZK
Answered
How does the presence of a catalyst affect an equilibrium system?
A) It lowers the activation energy of the forward reaction.
B) It lowers the activation energy for both reactions.
C) It lowers the activation energy of the reverse reaction.
D) It has no effect.
A) It lowers the activation energy of the forward reaction.
B) It lowers the activation energy for both reactions.
C) It lowers the activation energy of the reverse reaction.
D) It has no effect.
On May 10, 2024
B