SP
Sunny Pastagia
Answers (9)
SP
Answered
Which of the following correctly expresses the standard Gibbs free energy change of a reaction in terms of the changes in enthalpy and entropy?
A) ΔG° = ΔH° - TΔS°
B) ΔG° = ΔH° + TΔS°
C) ΔG° = ΔS° - TΔH°
D) ΔG° = ΔS° + TΔH°
E) none of the above
A) ΔG° = ΔH° - TΔS°
B) ΔG° = ΔH° + TΔS°
C) ΔG° = ΔS° - TΔH°
D) ΔG° = ΔS° + TΔH°
E) none of the above
On Aug 04, 2024
A
SP
Answered
________ is the use of an optically active reagent or catalyst to convert an optically inactive starting material into an optically active product.
A) Asymmetric induction
B) Racemization
C) Optical reduction
D) Meso effection
E) Chiralization
A) Asymmetric induction
B) Racemization
C) Optical reduction
D) Meso effection
E) Chiralization
On Aug 01, 2024
A
SP
Answered
In the reaction 4 Fe + 3 O2 → 2 Fe2O3, how much oxygen is needed to react completely with 19.83 g of iron?
On Jul 07, 2024
8.51 g
SP
Answered
What is the term for two molecules with the same formula but two different structures?
On Jun 25, 2024
Isomers
SP
Answered
Which of the following are components of the average fingerprint?
A) Water
B) Organic acids
C) Inorganic ions
D) All of the above
A) Water
B) Organic acids
C) Inorganic ions
D) All of the above
On Jun 04, 2024
D
SP
Answered
Draw a structure for the expected product of the following reaction. 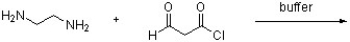

On May 30, 2024
SP
Answered
According to a phase graph such as that in Worked Example 9, what happens to the temperature of water from the time the first drop of liquid forms from ice to the time the last bit of ice melts?
A) It continues to rise slowly.
B) It stays the same.
C) It actually decreases slowly.
D) There is not enough information on the graph to tell.
A) It continues to rise slowly.
B) It stays the same.
C) It actually decreases slowly.
D) There is not enough information on the graph to tell.
On May 26, 2024
B
SP
Answered
What does the kinetic theory of gases state about the kinetic energy and temperature of the particles in a gas?
A) Average kinetic energy is inversely proportional to the temperature of the gas.
B) Average kinetic energy is proportional to the temperature of the gas.
C) Average potential energy is proportional to the temperature of the gas.
D) Average potential energy is inversely proportional to the temperature of a gas.
A) Average kinetic energy is inversely proportional to the temperature of the gas.
B) Average kinetic energy is proportional to the temperature of the gas.
C) Average potential energy is proportional to the temperature of the gas.
D) Average potential energy is inversely proportional to the temperature of a gas.
On May 23, 2024
B
SP
Answered
TNT has what kind of oxygen balance?
A) Positive
B) Neutral
C) Negative
D) Not enough information to tell
A) Positive
B) Neutral
C) Negative
D) Not enough information to tell
On May 19, 2024
C