ZK
Zybrea Knight
Answers (12)
ZK
Answered
Which of the following statements correctly describes the contribution of 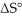 to
to 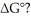
A) The entropy term makes a greater contribution to ΔG° at low temperatures.
B) The entropy term makes a greater contribution to ΔG° at high temperatures.
C) The entropy term makes a greater contribution to ΔG° in exothermic reactions.
D) The entropy term makes a greater contribution to ΔG° in endothermic reactions.
E) The entropy term always makes a more significant contribution to ΔG° than does the enthalpy term.
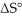 to
to 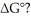
A) The entropy term makes a greater contribution to ΔG° at low temperatures.
B) The entropy term makes a greater contribution to ΔG° at high temperatures.
C) The entropy term makes a greater contribution to ΔG° in exothermic reactions.
D) The entropy term makes a greater contribution to ΔG° in endothermic reactions.
E) The entropy term always makes a more significant contribution to ΔG° than does the enthalpy term.
On Aug 04, 2024
B
ZK
Answered
In a carbon atom, the 2s and 2p orbitals are the same energy.
On Jul 12, 2024
False
ZK
Answered
The protons labeled Ha in the structure below generally show up as a triplet, even through there are three neighboring protons. The coupling constant is approximately 7 Hz. What does this suggest about the size of the coupling constant for the aldehydic proton? 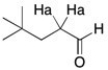

On Jul 09, 2024
It suggests that the coupling constant of the aldehydic proton is very small or even 0Hz.
ZK
Answered
What is the net change during a beta emission?
A) Loss of one proton, gain of one neutron
B) Loss of one neutron, gain of one proton
C) Loss of one neutron, gain of one electron
D) Loss of one electron, gain of one proton
A) Loss of one proton, gain of one neutron
B) Loss of one neutron, gain of one proton
C) Loss of one neutron, gain of one electron
D) Loss of one electron, gain of one proton
On Jun 12, 2024
B
ZK
Answered
In the reaction below, label each reactant as a nucleophile or an electrophile.
CH3COO- + O2S(OCH3)2 → CH3COOCH3 + CH3OSO3-
CH3COO- + O2S(OCH3)2 → CH3COOCH3 + CH3OSO3-
On Jun 09, 2024
CH3COO-, nucleophile
O2S(OCH3)2, electrophile
O2S(OCH3)2, electrophile
ZK
Answered
Draw the Lewis structure of acetic acid, CH3CO2H, including all non-bonding lone pairs.
On Jun 07, 2024
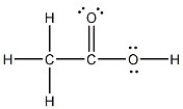
ZK
Answered
How does epicatechin affect muscle cells?
On May 13, 2024
It appears to increase the number of mitochondria.
ZK
Answered
What do all alkynes have in common?
On May 12, 2024
At least one carbon-carbon triple bond